Describe the Polarity of a Water Molecule
One of waters important properties is that it is composed of polar molecules. As discussed previously we can describe a compound with a molecular formula in which the subscripts indicate the actual numbers of atoms of each element in a molecule of the compound.
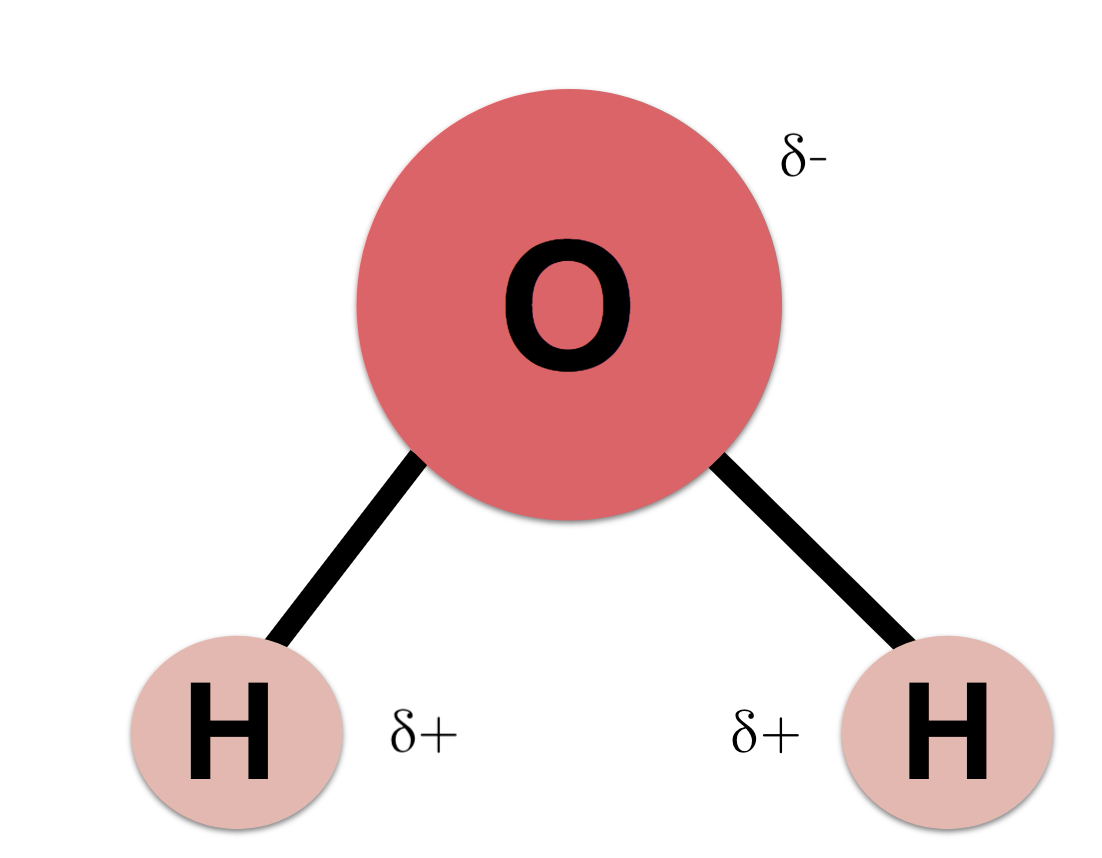
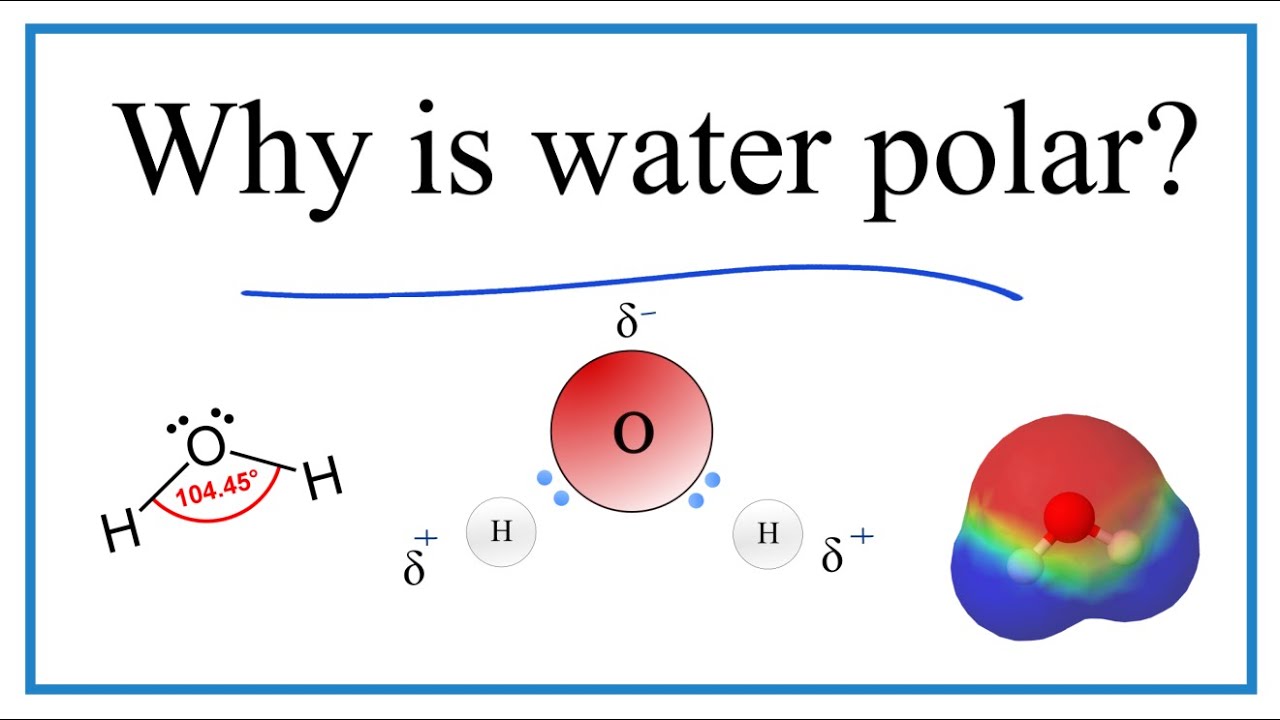
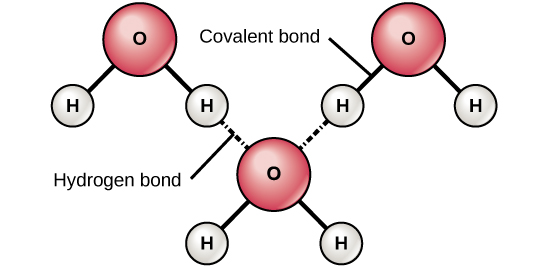
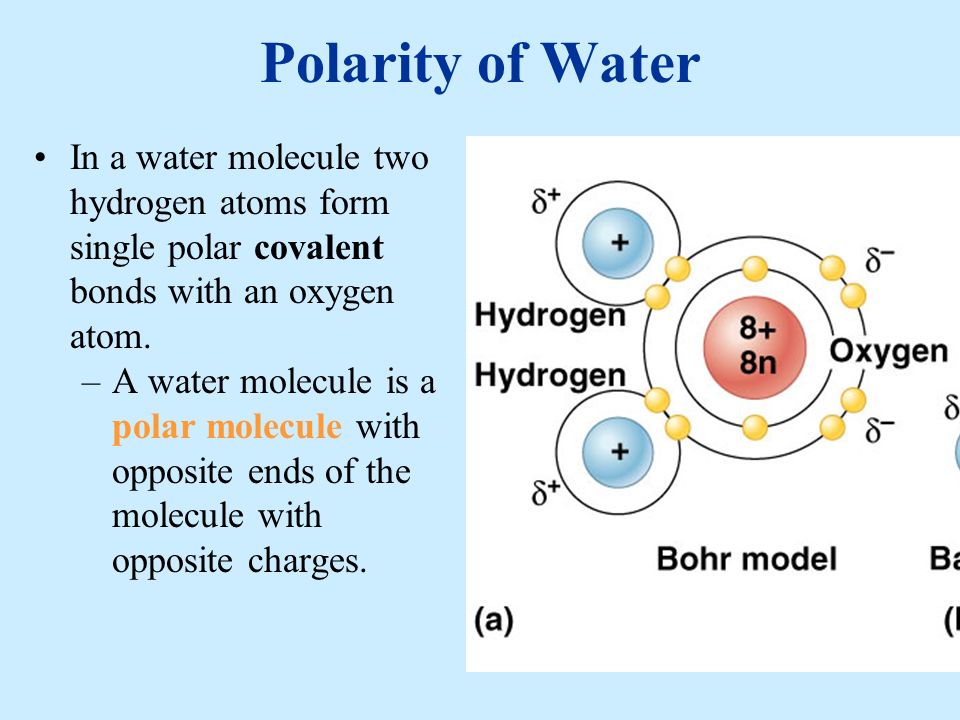
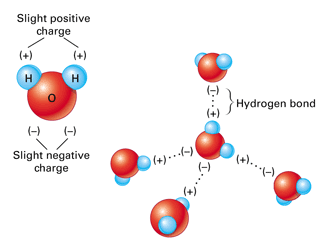
The two hydrogen atoms and one oxygen atom within water molecules H 2 O form polar covalent bonds.
. Describe the TWO factors that determine whether a molecule is polar or non-polar. It is linear in shape and has no polarity. The permanent dipole in water is caused by oxygen s tendency to draw electrons to itself ie.
___ c A molecule cannot be non-polar if it contains only polar bonds. It is important that rain and water are not absorbed through the leaves as this would disrupt the flow of nutrients which rely on the passage of water from root to leafIf the water were allowed to travel by osmosis through the cell membrane and into the leaf it would change the osmotic pressure in the leaves and. In many cases the molecular formula of a substance is derived from experimental determination of both its empirical formula and its molecular mass the sum of atomic masses for all atoms.
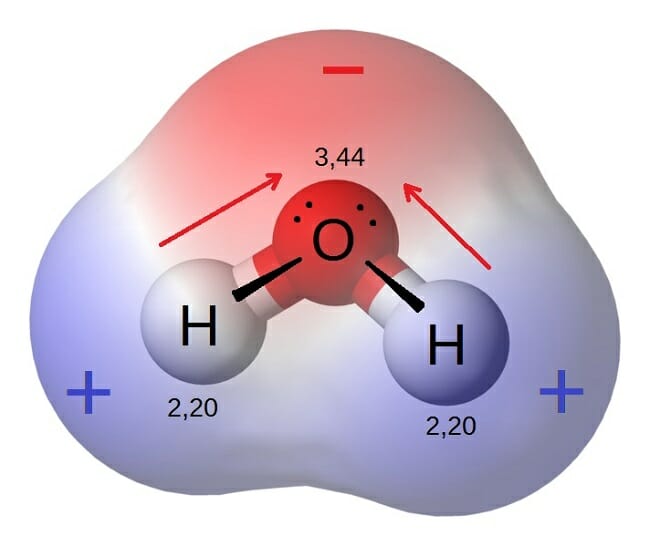
___ T ___ d A molecule consisting of 2 atoms is always linear. Molarity - PhET Interactive Simulations. Oxygen is more electronegative than hydrogen.
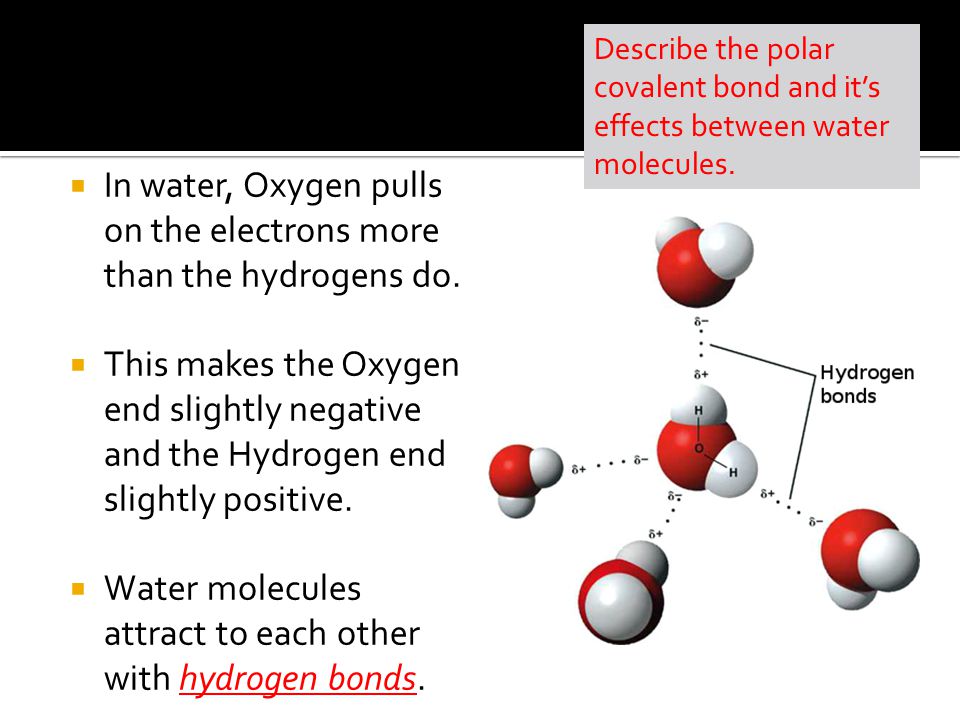
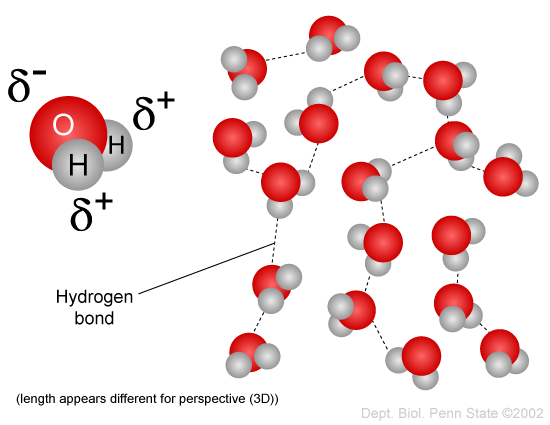
While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to waters properties of. Many plants have hydrophobic coatings on their leaves. As a result of waters polarity each water molecule attracts other water molecules because of the opposite charges between them.
The 10 electrons of a water molecule are found more regularly near the oxygen atoms nucleus which contains 8 protons. ___ T __ e Polar molecules have stronger attractions for each other than non-polar molecules have. As a result oxygen has a slight negative charge δ-.
In concluding remarks to sum up this entire article I3- is a polyatomic ion that has 22 valence electrons 3 lone pairs 2 bond pairs and sp3d hybridization. The two hydrogen atoms and one oxygen atom within water molecules H 2 O form polar covalent bonds. However if you have to describe the ion you can use the phrase the like a polar molecule because I3- is soluble in water.
Why are the bonds in a water molecule polar covalent and how does that affect the interactions between water molecules.
Why Is Water H2o A Polar Molecule Youtube

The Structure Of Water Chemistry For Non Majors
Polar Molecule Definition And Examples Biology Dictionary
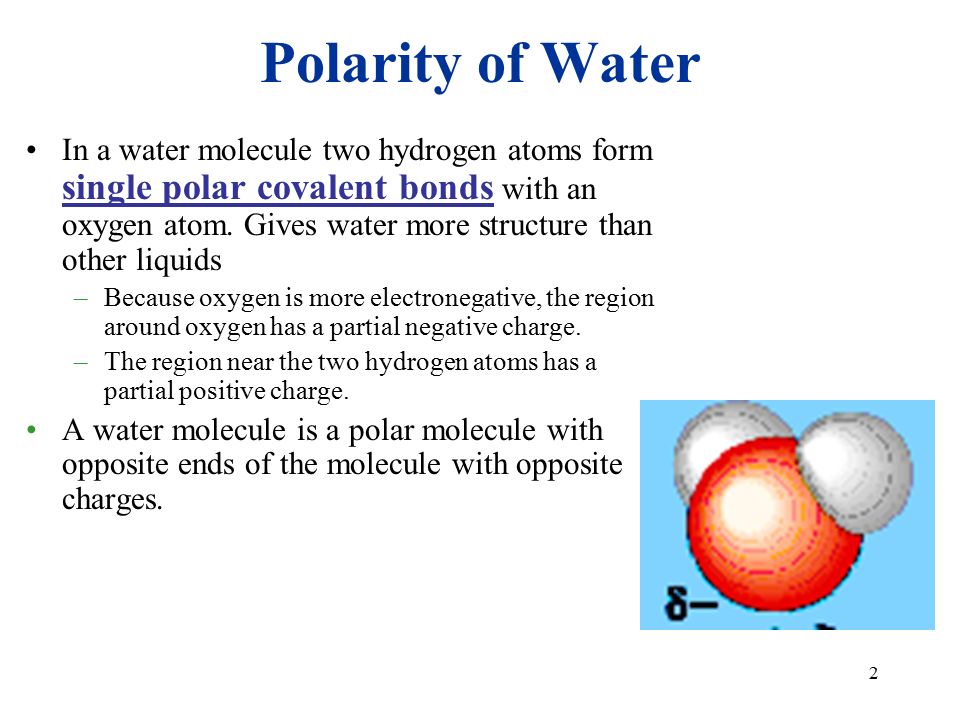
1 2 Polarity Of Water In A Water Molecule Two Hydrogen Atoms Form Single Polar Covalent Bonds With An Oxygen Atom Gives Water More Structure Than Other Ppt Download
Why Life Depends On Water Biology For Non Majors I
What Are Some Examples Of A Polarized Molecule Quora

The Structure And Properties Of Water Introduction To Chemistry
What Is Polar Nature Of Water Quora

Lesson Summary Water And Life Article Khan Academy
Water Molecule Structure Course Hero

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

O H H Water H 2 O Water Is A Polar Molecule This Means That A All Atoms Have Equal Electrical Charges B A Water Molecule Is Linear Ppt Download
Water Molecule Dipole Moment The Polarity Of Water Affects Its Properties Causes Water To Remain Liquid At Higher Temperature Permits Ionic Compounds Ppt Download

Why Is Water Considered A Polar Molecule Quora
Water Describe Water 46nbtop How Would You Describe Water To Someone Who Had Never Seen It Before You Might Say That Pure Water Has No Color No Taste Ppt Download

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
Comments
Post a Comment